Italian National Agency for New Technologies, Energy and Sustainable Economic Development

Environment: ENEA and the University of Pavia develop new material absorbing pollutants from water
A joint team from ENEA and University of Pavia has developed an innovative material which can capture silver nanoparticles dispersed in water. The outcomes of the research were published in the scientific journal "Molecules"[1].
Ultrafine silver particles, smaller than 100 nanometers in size, exihibit disinfectant properties that make them one of the most used products in nanotechnology, with an annual production of around 500 tonnes. Their use in medical-health devices, household appliances, furniture, toothbrushes and clothes, involves their dispersion in water, where they can remain intact for many days.
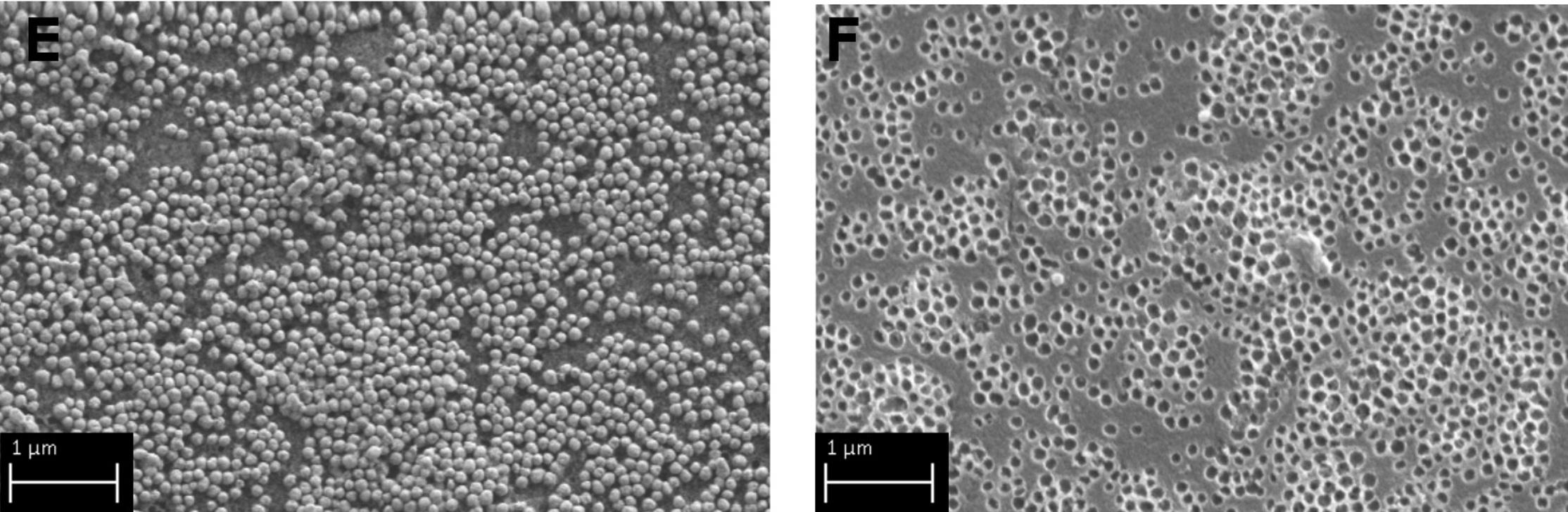
The new material, based on a harmless and inert compound used to make glass- silica- treated with a so-called nanoimprinting technique[2], allows to obtain cavities the same size as the silver nanoparticles to be removed from water. “This study focused on the characterization of silica monoliths, before and after nanoparticles removal,” explained Maria Lucia Protopapa, researcher at the Laboratory of Functional Materials and Technologies for Sustainable Applications at the ENEA Brindisi Research Center. “In particular - she said - we conducted chemical, thermal and morphological analyses using high-resolution scanning electron microscopy and, above all, porosimetric analyses to obtain information on the size and number of pores on the surface of the silica”.
“Thanks also to strong attractive physical forces, the nanoparticles enter the silica cavities of corresponding dimensions. Once they have adhered to the much larger silica fragments, they can be easily removed from the water,” explained Professor Piersandro Pallavicini at the Chemistry Department of the University of Pavia and research coordinator.
Laboratory tests have shown that this material can effectively capture silver nanoparticles from water: one gram of nanoimprinted silica (the amount contained in a silica disk 3 centimeters in diameter and half a centimeter thick) can remove over 4 milligrams of silver nanoparticles, approximately one million billion nanoparticles. Therefore, nanoparticle-imprinted silica could be employed on a large scale to recover other types of nanoparticles, also from polluted wastewater.
The collaboration with the University of Pavia is part of a broader agreement between ENEA and the Lombardy Region for the valorisation of human resources, with direct impacts on the research, innovation and territory system. This agreement led to 19 three-year PhD scholarships (2019-2022) and the creation of three ENEA laboratories in Lombardy, two at the “Kilometro Rosso” Scientific and Technological Park and one at the University of Brescia.
For more information please contact:
Notes
[1] The study is part of a PhD in Chemical and Pharmaceutical Sciences and Industrial Innovation funded by the Lombardy Region.
[2] Silver nanoparticles prepared by the laboratories of the University of Pavia in the desired dimensions are dispersed in a liquid solution from which, by simple drying, solid silica (called gel) is formed, with the nanoparticles trapped inside. These are then dissolved with a green procedure (for example, by exposure to air in the presence of certain amino acids), the silver is released, recovered and recycled and a solid silica is obtained with cavities identical to the nanoparticles dissolved. At this point the material is ready to selectively capture and remove new silver nanoparticles dispersed in water.